44 structure function claims on dietary supplement labels
Dietary Supplement Labeling Guide: Chapter VI. Claims - FDA 1 Apr 2005 — A health claim is an explicit or implied characterization of a relationship between a substance and a disease or a health-related condition. Dietary supplement - Wikipedia Definition. In the United States, the Dietary Supplement Health and Education Act of 1994 provides this description: "The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or ...
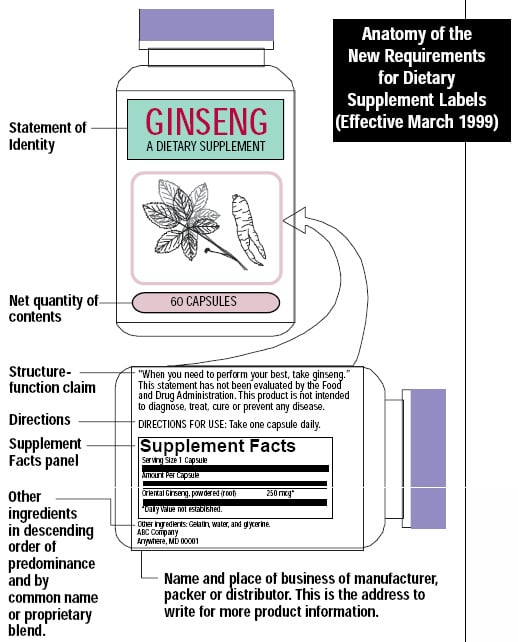
Small Entity Compliance Guide on Structure/Function Claims What are structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) ... Dietary supplement labels or labeling may, subject to the ...

Structure function claims on dietary supplement labels
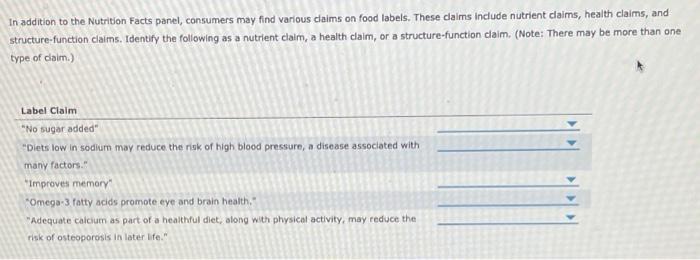
Structure/Function Claims Fail To Meet Federal Requirements ... by DR Levinson · 2012 · Cited by 4 — about a specific type of claim—called a structure/function claim—that manufacturers may use on dietary supplement labels. Manufacturers have used these ...31 pages Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Label Claims for Food & Dietary Supplements - FDA 7 Mar 2022 — Health claims, nutrient content claims, and structure/function claims used on food and dietary supplement labels.
Structure function claims on dietary supplement labels. Vitamin E - Wikipedia The U.S. Food and Drug Administration initiated a process of reviewing and approving food and dietary supplement health claims in 1993. Reviews of petitions results in proposed claims being rejected or approved. If approved, specific wording is allowed on package labels. In 1999, a second process for claims review was created. Dietary Supplements for Weight Loss - Health Professional ... Approximately 15% of U.S. adults have used a weight-loss dietary supplement at some point in their lives; more women report use (21%) than men (10%) . Americans spend about $2.1 billion a year on weight-loss dietary supplements in pill form (e.g., tablets, capsules, and softgels) [ 9 ], and one of the top 20 reasons why people take dietary ... Structure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ... Understanding Dietary Supplement Claims 1. Structure/function claims are the most common, permissible claim used for dietary supplements. DSHEA established special requirements for structure/function ...
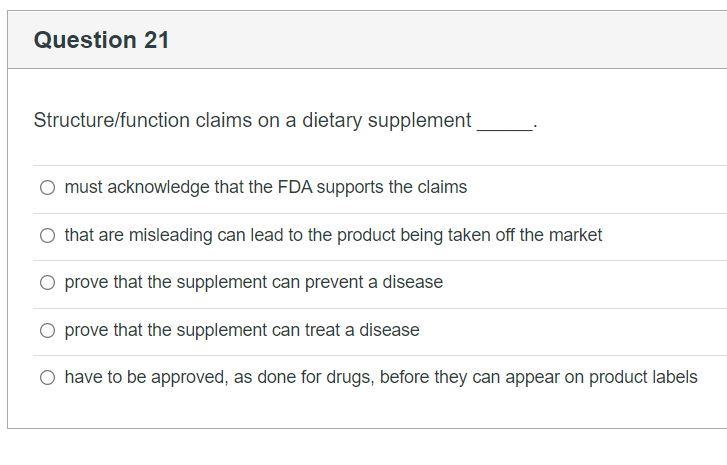
Guidance on Substantiation for Dietary Supplement Claims Please see the Federal Register of January 6, 2000 (65 FR 1000, codified at 21 CFR 101.93) for the final rule defining structure/function claims for dietary supplements and the January 9, 2002 ... Permissible vs. Impermissible Structure/Function Claims for ... Structure/Function Claims for Dietary Supplements ... What is a Structure/Function Claim? ... are permissible if the labeling taken as a whole does.28 pages Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for using structure/function claims and two related types of dietary ... Structure/Function Claim Notification for Dietary Supplements 16 Dec 2019 — The name and address of the manufacturer, packer, or distributor of the dietary supplement product;; the text of the statement that is being ...
Label Claims for Food & Dietary Supplements - FDA 7 Mar 2022 — Health claims, nutrient content claims, and structure/function claims used on food and dietary supplement labels. Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Structure/Function Claims Fail To Meet Federal Requirements ... by DR Levinson · 2012 · Cited by 4 — about a specific type of claim—called a structure/function claim—that manufacturers may use on dietary supplement labels. Manufacturers have used these ...31 pages










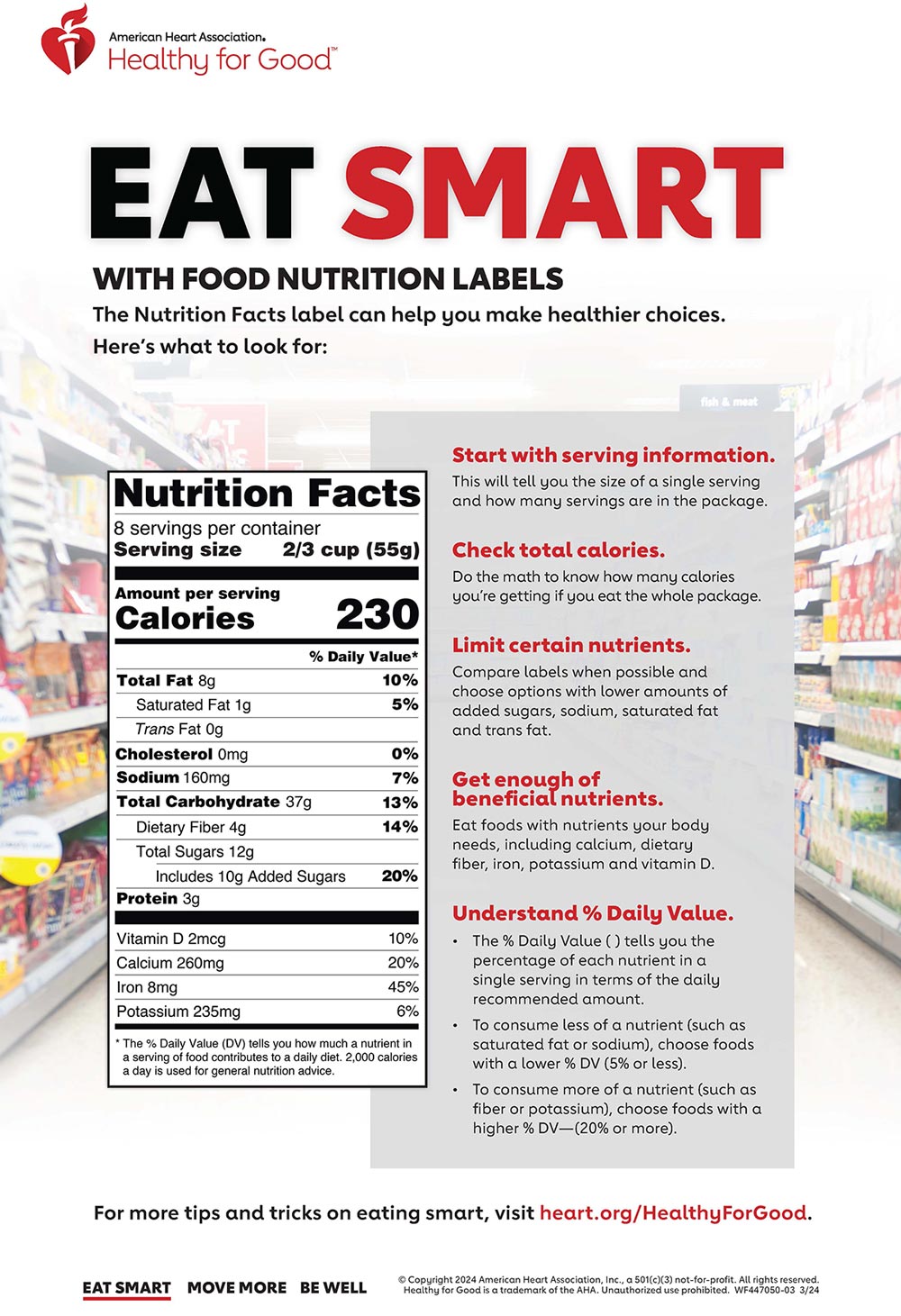

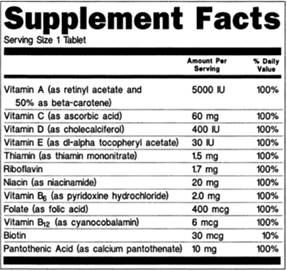







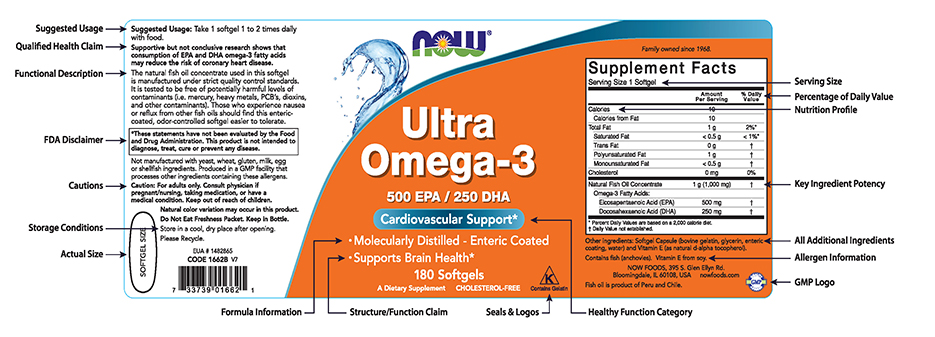

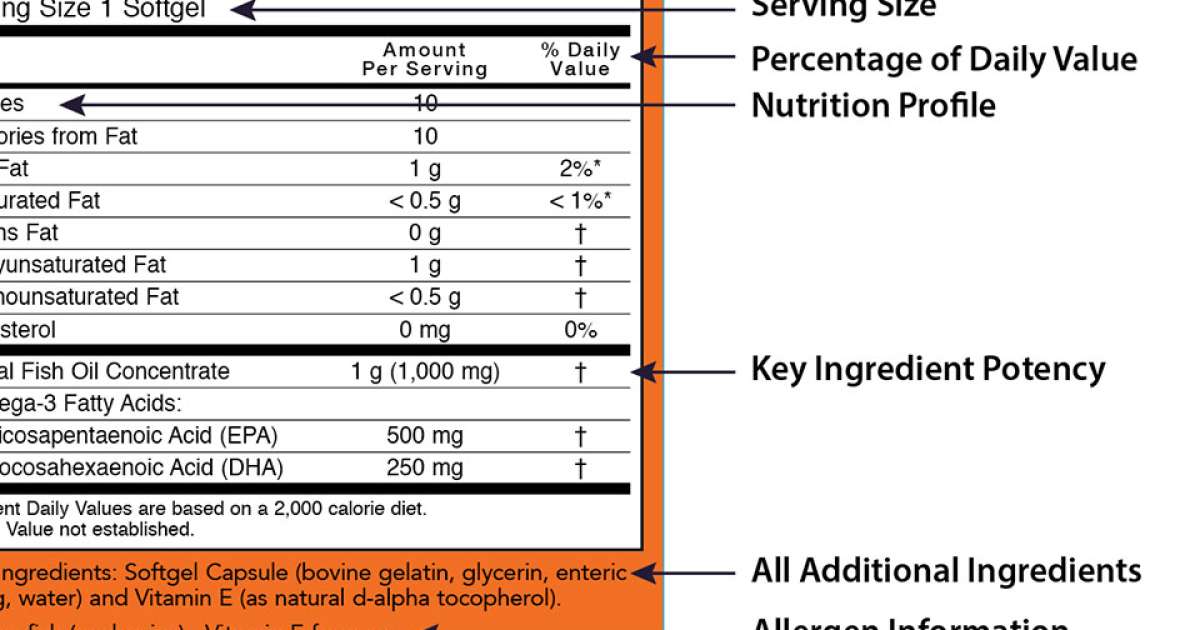










Post a Comment for "44 structure function claims on dietary supplement labels"