38 common auxiliary labels for drugs
› sites › defaultISMP List of High-Alert Medications the ordering, storage, preparation, and administration of these products; improving access to information about these drugs; limiting access to high-alert medications; using auxiliary labels; employing clinical decision support and automated alerts; and using redundancies such as automated or independent double checks when necessary. › en › health-canadaGuidance Document: Labelling of Pharmaceutical Drugs for ... Nov 01, 2013 · The purpose of this document is to provide guidance to sponsors to facilitate compliance with the labelling requirements pursuant to sections 3, 9, and 10 of the Food and Drugs Act as well as related provisions of the Food and Drug Regulations, the Controlled Drugs and Substances Act, and its related Regulations including the Narcotic Control Regulations, Parts G and J of the Food and Drug ...
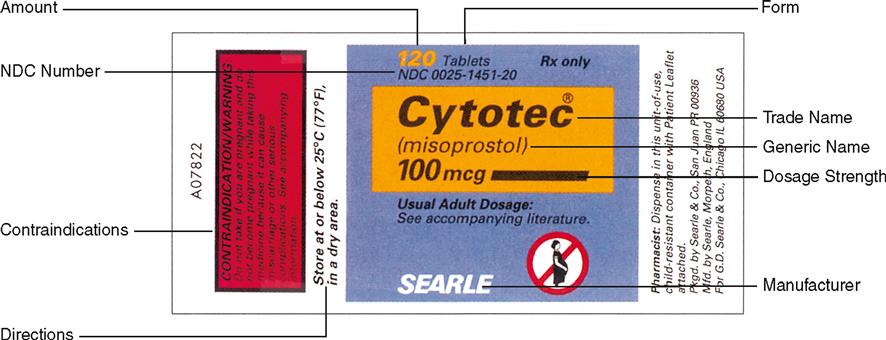
› PharmacyAuxiliaryLabelsPharmacy Auxiliary Labels Packaged in packs of 1000, our Auxiliary Labels contain Warnings, Dietary information, instructions for Routes of Administrations, Cautionary details…even messages in Spanish and French. All labels measure 3/8” x 1 ½” and are brightly colored so they call attention to themselves.

Common auxiliary labels for drugs
ISMP List of High-Alert Medications the ordering, storage, preparation, and administration of these products; improving access to information about these drugs; limiting access to high-alert medications; using auxiliary labels; employing clinical decision support and automated alerts; and using redundancies such as automated or independent double checks when necessary. (Note ... VelveteenDuck Welcome to the VelveteenDuck! The most reliable source of guides for Drakensang Online. Investigational Drugs: Strategies for Sponsors, FDA, and … 03/05/2018 · Auxiliary labels and highlights should be applied before the drugs are added to stock or dispensed to patient care areas. To prevent mix-ups, separate investigational drugs for single patient compassionate use from the main study supplies, and separate commercially available products used in clinical trials from the main pharmacy supplies.
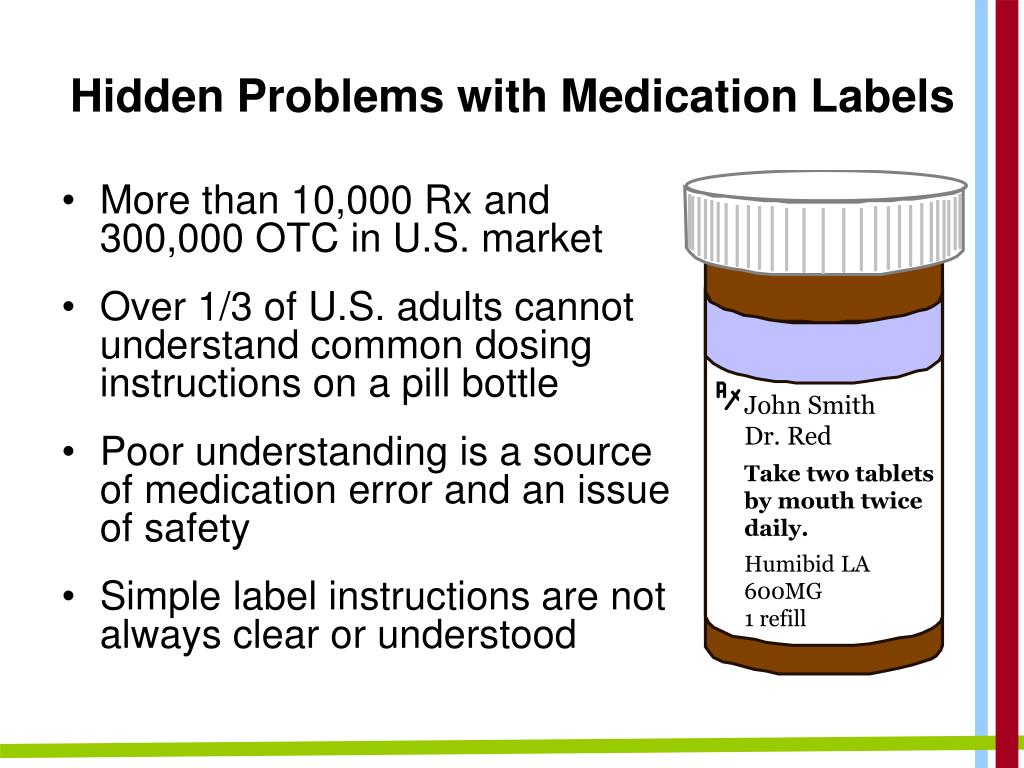
Common auxiliary labels for drugs. Auxiliary labels at its finest. Apr 25, 2022. ... Lack of common sense, Illegal drugs. There’s safer drugs than Superman Pills for your problems. OWNER’S MANUAL POWERSPORTS UT400 - Tractor Supply … Auxiliary DC Jack 4-27 . Pre-Operation Checks. 5. 5-1 . Brakes 5-2 Front and Rear Brakes Brake Pedal 5-2 Brake Fluid Level 5-3 Brake Operation 5-3 Fuel 5-4 . 2 3 1 . Coleman UT400 Owner Manual. Engine Oil 5-6 Coolant 5-6 Final Gear Oil 5-7 Differential Gear Oil 5-7 Throttle Pedal 5-8 Throttle Freeplay 5-9 Throttle Freeplay Inspection 5-9 Throttle Freeplay Adjustment 5-9 … Prescription Drug Labeling Medication Errors: A Big Deal for ... 20/07/2006 · In a modern study, conducted by Shrank et al. and they showed after gathered data from identically written prescriptions filled for four commonly prescribed drugs (Atorvastatin, Alendronate, Trimethoprim-Sulfamethoxazole, and Ibuprofen) in six different pharmacies (two chains, two independent, and two grocery stores) in four diverse cities. The evaluation of the … Shop by Category | eBay Shop by department, purchase cars, fashion apparel, collectibles, sporting goods, cameras, baby items, and everything else on eBay, the world's online marketplace
11 Typical Pharmacy Technician Duties Ensure proper storage of drugs in adherence to regulations that apply to a pharmacy. While stock taking, expired medications have to be checked for. Medications nearing expiry require to be shipped back to the pharmaceutical company. Supplies such as pill containers, bags and labels need to kept in an organized manner and ordered whenever required. 8. Enter Patient Data: … Guidance Document: Labelling of Pharmaceutical Drugs for … 01/11/2013 · The purpose of this document is to provide guidance to sponsors to facilitate compliance with the labelling requirements pursuant to sections 3, 9, and 10 of the Food and Drugs Act as well as related provisions of the Food and Drug Regulations, the Controlled Drugs and Substances Act, and its related Regulations including the Narcotic Control Regulations, … Pharmacy Auxiliary Labels Packaged in packs of 1000, our Auxiliary Labels contain Warnings, Dietary information, instructions for Routes of Administrations, Cautionary details…even messages in Spanish and French. All labels measure 3/8” x 1 ½” and are brightly colored so they call attention to themselves. Because these labels have been in use for years, the graphics depicting what’s … Pharmacy and Medical Terminology Pharmacy Techs Need to … 11/05/2020 · Auxiliary label – Additional labels placed on prescription packaging that provide supplementary information, various warnings, routes of administrations, etc. 2 AWP (average wholesale price) – Found in the pharmaceutical reference book also called the Red Book, the AWP of a drug is the average price at which drugs are purchased wholesale. 2
› resources › investigational-drugsInvestigational Drugs: Strategies for Sponsors, FDA, and ... May 03, 2018 · Auxiliary labels and highlights should be applied before the drugs are added to stock or dispensed to patient care areas. To prevent mix-ups, separate investigational drugs for single patient compassionate use from the main study supplies, and separate commercially available products used in clinical trials from the main pharmacy supplies. › n › all-categoriesShop by Category | eBay Shop by department, purchase cars, fashion apparel, collectibles, sporting goods, cameras, baby items, and everything else on eBay, the world's online marketplace › pmc › articlesPrescription Drug Labeling Medication Errors: A Big Deal for ... Jul 20, 2006 · The problem extends to the auxiliary sticker labels that provide accompanying warnings and directions for use of the medicine.[29,31] Other studies demonstrated that over half (53%) of patients, especially those with limited literacy, had difficulty interpreting text and icons usually used on auxiliary warning instructions. Investigational Drugs: Strategies for Sponsors, FDA, and … 03/05/2018 · Auxiliary labels and highlights should be applied before the drugs are added to stock or dispensed to patient care areas. To prevent mix-ups, separate investigational drugs for single patient compassionate use from the main study supplies, and separate commercially available products used in clinical trials from the main pharmacy supplies.
VelveteenDuck Welcome to the VelveteenDuck! The most reliable source of guides for Drakensang Online.
ISMP List of High-Alert Medications the ordering, storage, preparation, and administration of these products; improving access to information about these drugs; limiting access to high-alert medications; using auxiliary labels; employing clinical decision support and automated alerts; and using redundancies such as automated or independent double checks when necessary. (Note ...












Post a Comment for "38 common auxiliary labels for drugs"