42 when are supplier labels required to be updated
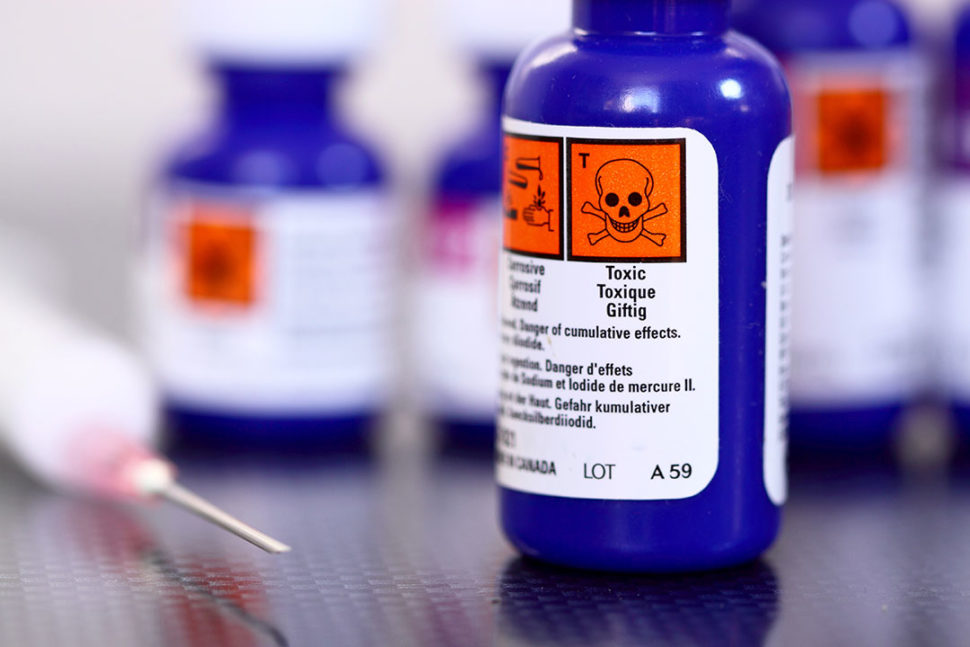
European Union Product Labeling Requirements: A Complete Guide Clothing and other products containing a minimum of 80% by weight of textile fibers must be labeled with the correct fiber composition (e.g. 100% Cotton or 100% Polyester). Further, the label must be permanent, which means it must either be attached to the clothing item or printed. A sticker is not enough. Product Examples T-shirts Underwear Supplier and Workplace Labels - Sharp Academy There are two main types of WHMIS labels: supplier labels and workplace labels. A supplier label is provided or affixed (attached) by the supplier and will appear on all hazardous products received at a workplace in Canada. If the hazardous products are always used in the container with the supplier label, no other label is required.
FDA Announces Temporary Food Labeling During COVID-19 Pandemic Constituent Update. May 22, 2020. The U.S. Food and Drug Administration is issuing a guidance document to provide additional temporary flexibility in food labeling requirements to manufacturers ...
When are supplier labels required to be updated
WHMIS 2015 - Labels : OSH Answers Labels will be required to be updated within 180 days of the supplier being aware of the new information. If you purchase a product within this 180 day time period, the supplier must inform you of the changes, and the date they became available, in writing. Quality System Regulation Labeling Requirements | FDA When a manufacturer modifies a device, the manufacturer must also review the labeling to make certain that it reflects current revisions and specifications. Some manufacturers identify labeling... Labelling and packaging - ECHA Labelling and packaging. Once the hazardous properties of a substance or mixture have been identified, they need to be classified accordingly. Manufacturers, importers, downstream users and distributors, as well as producers and importers of certain specific articles, must communicate the identified hazards to the other actors in the supply ...
When are supplier labels required to be updated. Compliance FAQs: Packaging and Labeling in the US | NIST The UPLR requires that consumer packaging bear a label specifying the following: the name and place of business of the manufacturer, packer, or distributor. the net quantity of contents in terms of weight or mass measure, or numerical count in a uniform location upon the principal display panel. Q&As - ECHA Based on this provision, it is possible to place the name, address and telephone number of more than one supplier on the label. The contact details of any supplier in the supply chain are allowed to be included on the label. More information can be found in the Guidance on labelling, section 4.1, and in the CLP FAQ on labelling (unique ID 242). whmis workplace labels are required when - Voicemailcampaign What are the 6 pieces of information required on a supplier label. This section does not require employers to use WHMIS symbols and a hatched border when preparing workplace labels. In WHMIS 2015 the labels of hazardous products must display the information elements shown below. How Often Should You Update Your Safety Data Sheets ... As for updating labels with new, 'significant information,' OSHA says, "Chemical manufacturers, importers, distributors, or employers who become newly aware of any significant information regarding the hazards of a chemical shall revise the labels for the chemical within six months of becoming aware of the new information, and shall ensure that labels on containers of hazardous chemicals shipped after that time contain the new information."
Hazard communication effective dates and labels ... Response: HCS 2012 requires the following, per paragraph 1910.1200(f)(11): "Chemical manufacturers, importers, distributors, or employers who become newly aware of any significant information regarding the hazards of a chemical shall revise the labels for the chemical within six months of becoming aware of the new information, and shall ensure that labels on containers of hazardous chemicals shipped after that time contain the new information. If the chemical is not currently produced or ... Prop 65: The Updated Rules You Need to Be Following - Avery New warnings with more specific information for exposure from certain products and places are now required for Prop 65 signs and labels. These include food and drinks (alcoholic and non), prescription drugs, dental care products, furniture and wood dust, diesel engines, automobiles and recreational vessels, parking garages, amusement parks, gas stations and auto repair shops, designated smoking areas and hotels. Labeling requirements of hazardous chemicals ... MAKE THE RATING AND LABELING OF HAZARDOUS PRODUCTS, as required by the OSHA Hazard Communication Standard of August 24, 1987, (29 CFR 1910.1200) THE RESPONSIBILITY OF THE MANUFACTURER OR DISTRIBUTOR RATHER THAN HEALTH CARE PERSONNEL. The HCS establishes labeling requirements that must be met by the chemical manufacturer, importer or distributor. Medical Device Labeling Changes and Challenges - EU MDR Medical Device Labeling Changes and Challenges - EU MDR. Implementation of the EU MDR can be complex and challenging. With the enforcement deadline being just around the corner, medical device manufacturers must execute the new format of labeling information with utmost priority and caution. The following is the twelfth in the series of EU ...
FAQs: Updating and Managing Safety Data Sheets | TotalSDS® And for updating labels with new, 'significant information,' OSHA says, "Chemical manufacturers, importers, distributors, or employers who become newly aware of any significant information regarding the hazards of a chemical shall revise the labels for the chemical within six months of becoming aware of the new information, and shall ensure that labels on containers of hazardous chemicals shipped after that time contain the new information." CE marking - GOV.UK The CE marking is required for many products. It: ... Last updated 31 December 2020 + show all updates. 31 December 2020. Added a section on choosing which conformity marking to use. Medicines: packaging, labelling and patient information ... approval of artwork for a new own-label supplier not previously known to us ... If a safety issue is identified the MA holder is required to ... Updated to reflect changes in regulations following ... EU - Labeling/Marking Requirements Starting on July 16, 2021, all CE marked products will need to have an EU address on the label. This also applies to products sold online. The name and address must appear on the product or the product's packaging so that customs and market surveillance authorities can have a contact person in case the product is suspected to present a risk.
GHS Labeling Requirements: The Definitive Guide [2021 ... June 1, 2015: Distributors were given six months to ship old inventory before they had to join chemical manufacturers in completing hazard reclassification and producing GHS labels and data sheets. December 1, 2015: The grace period for distributors ended and the companies had to begin complying with GHS requirements.
When Should You Update or Revise Your SDSs? Suppliers and employers must update SDSs and labels within 6 months since new information concerning a chemical is made available. Suppliers should also periodically review label and SDSs every 5 years even if no new information has been provided. Ref: GHS in Singapore. Australia. In Australia, the manufacturer or importer of the hazardous chemical must:
WHMIS 1988 - Labelling Requirements : OSH Answers When the material is moved into containers for resale or delivery out of your workplace, you must put a supplier label on each container. When the bulk material is used in your workplace (usually transferred into smaller containers), a workplace label is required on the containers.
How Often Do SDS Sheets Need to Be Updated/Replaced ... When you become newly aware of this information, you must revise the labels. Labels must happen within 6 months of acquiring the new information. Any chemical shipped after that 6-month timeframe must contain the new information. SDS should be modified in 3 months. Sometimes, the chemical isn't currently being imported or even produced.
United States Product Labeling Requirements: An Overview Bag Suffocation Warning labeling is required when selling products in certain types of plastic bags on Amazon, and also a legal requirement in California, New York, and other states. Note that the specific warning labeling requirements (e.g. the text printed on the plastic bag) differ depending on the following factors: Size of the bag opening
Information Elements Required on a WHMIS 2015 Label ... The only non-prescribed hazard and precautionary statements appear in Schedule 5 of the HPR. Labels must be updated to include significant new data - data which changes the classification of the product or ways to protect against the hazards presented by the hazardous product - within 180 days of becoming available.
Label your products | business.gov.au If you are an importer, manufacturer or supplier of energy efficiency regulated products in Australia, you may be required to display an energy rating label. The fuel consumption labelling standard requires a model specific fuel consumption label to be placed on the windscreens of all new vehicles up to 3.5 tonne gross vehicle mass.
Labelling and packaging - ECHA Labelling and packaging. Once the hazardous properties of a substance or mixture have been identified, they need to be classified accordingly. Manufacturers, importers, downstream users and distributors, as well as producers and importers of certain specific articles, must communicate the identified hazards to the other actors in the supply ...
Quality System Regulation Labeling Requirements | FDA When a manufacturer modifies a device, the manufacturer must also review the labeling to make certain that it reflects current revisions and specifications. Some manufacturers identify labeling...
WHMIS 2015 - Labels : OSH Answers Labels will be required to be updated within 180 days of the supplier being aware of the new information. If you purchase a product within this 180 day time period, the supplier must inform you of the changes, and the date they became available, in writing.









Post a Comment for "42 when are supplier labels required to be updated"